7667766266
enquiry@shankarias.in
Recently, Gambia declared that from July 1, 2023, it is running strict quality control checks on all pharma products imported from India due to contaminated drugs.
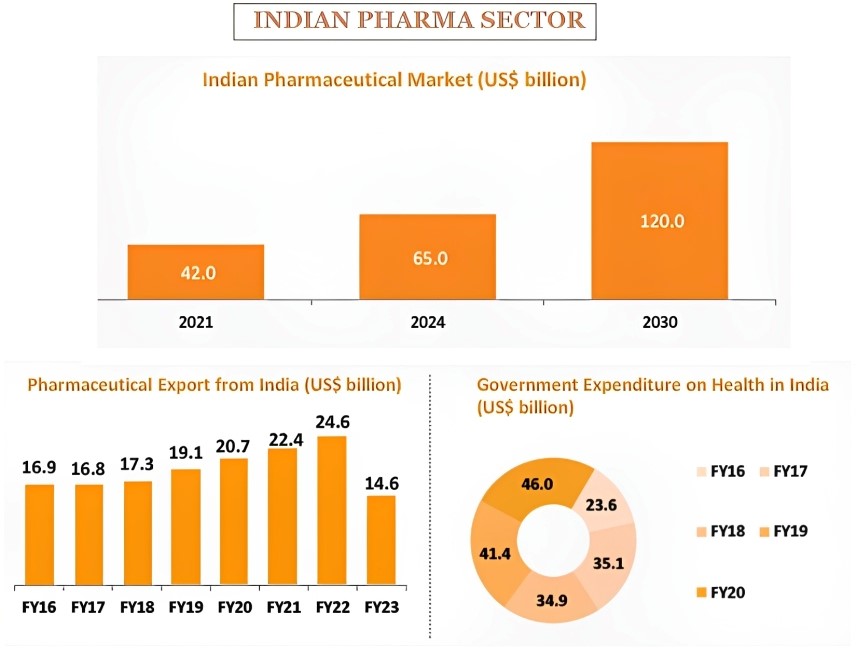
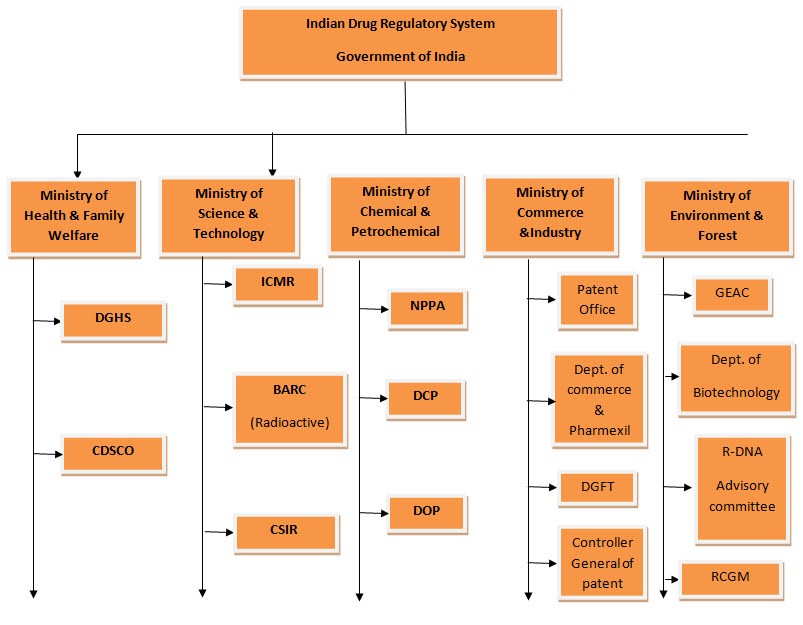
For example: Karnataka is working at nearly half its sanctioned capacity for drug inspectors.