7667766266
enquiry@shankarias.in
Doctors have been protesting on new guidelines for professional conduct to use generic names of medicines on the prescription instead of a particular brand name.
|
Picture of India’s Pharma Industry |
13 % of the global pharma market.
|
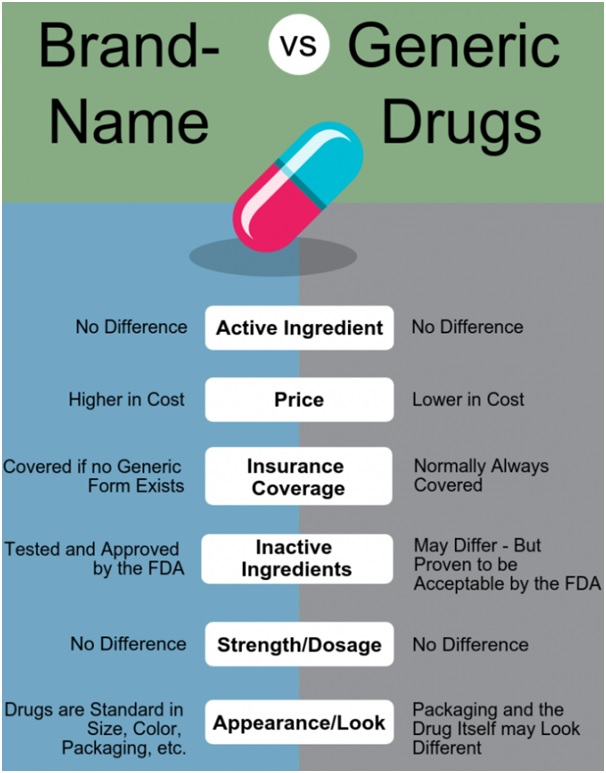
On an average, generic medicines are 30% to 80% cheaper than the branded versions and hence likely to bring down healthcare costs.
Indian Medical Association (IMA) is the largest body of doctors in the country
References