7667766266
enquiry@shankarias.in
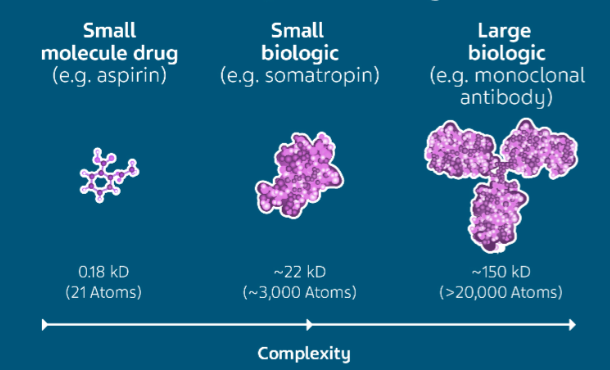
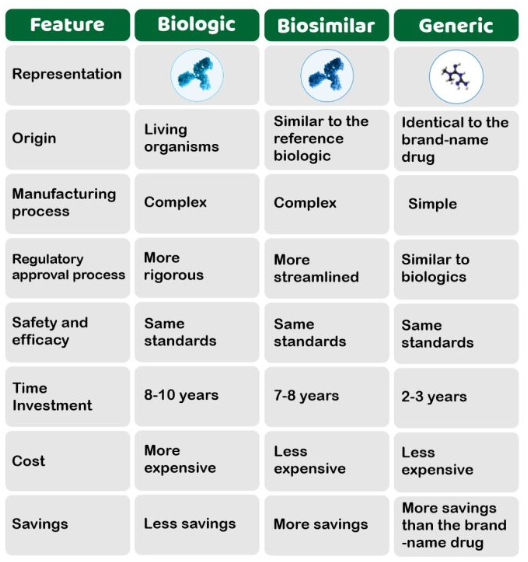
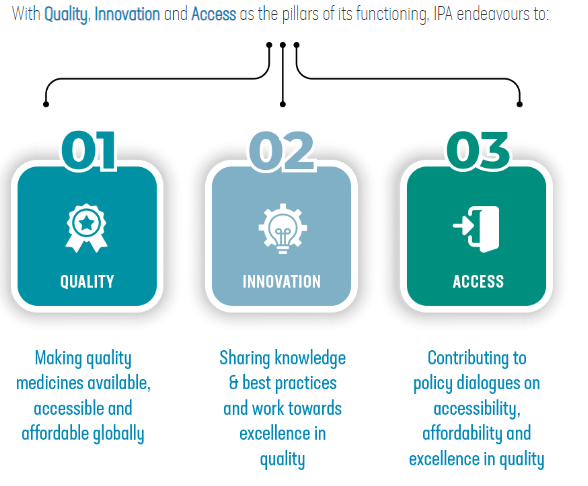
Recently, the biopharmaceutical alliance was launched to build a resilient supply chain in the biopharmaceutical sector.
|
India's Pharmaceutical Sector |
|