7667766266
enquiry@shankarias.in
Johnson & Johnson’s patent on bedaquiline expired recently which will allow generic manufacturers to supply this crucial drug for Tuberculosis.
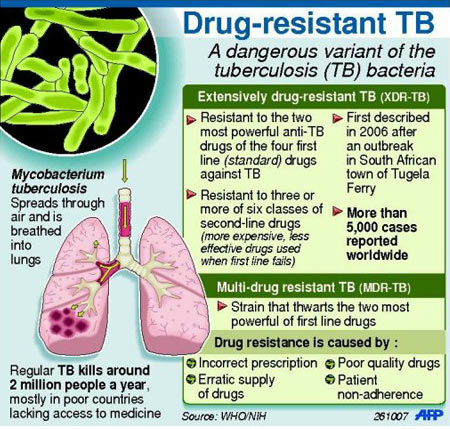
Each year, nearly half a million people develop drug-resistant TB and nearly 10.4 million people develop drug-sensitive TB.
To know more click - The Road to End Tuberculosis (TB)
|
Quick Facts |
|
References