7667766266
enquiry@shankarias.in
What is the issue?
Despite DCGI granting Emergency Use Authorisation (EUA) for two vaccines, uncertainty in vaccine availability remains because of the government’s indecision in granting indemnity.
What is a EUA?
What is the status of India’s vaccine program?
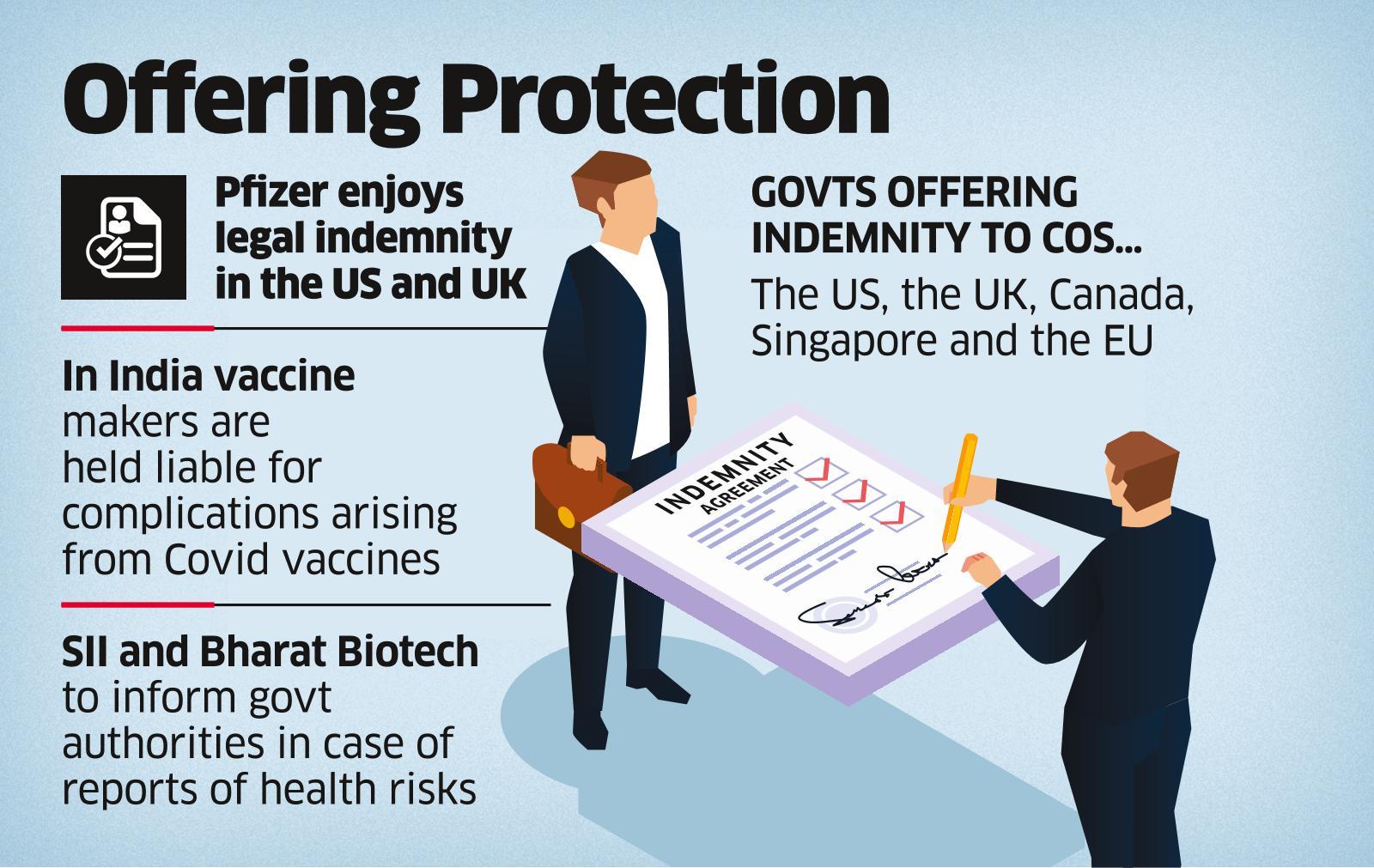
What is indemnity?
What is the International Practice?
What is the situation in India?
Why is the government hesitant on granting indemnity?
What are the existing safety mechanisms?
Class action suits - cases representing groups of people who have suffered from the same loss
What should the government do?
Source : The Hindu