7667766266
enquiry@shankarias.in
Mains (GS II): Issues relating to development and management of Social Sector/Services relating to Health, Education, Human Resources.
Recently, Karnataka government seeks corporate support for treating children battling rare and ultra rare diseases.
|
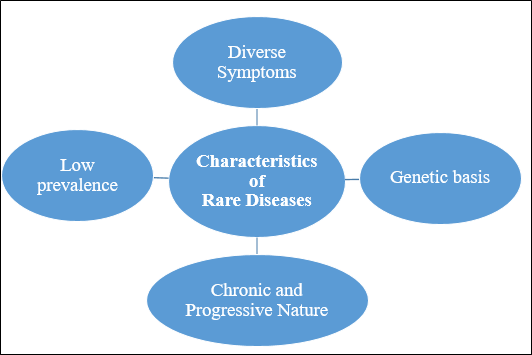
Status of Rare Disease in India |
|
Rare Disease Day was observed on the last day of February i.e., 28th February (or 29 in leap years).