Performance Grading Index
Ministry of Education releases report on Performance Grading Index 2.0 for States/UTs for the year 2021-22.
- Performance Grading Index (PGI) measures the performance of states/UTs in school education.
- Launched – In 2017-18 and so far, has been released up to the year 2020-21.
- PGI 2.0 - To align with the National Education Policy, 2020, and to monitor indicators relating to Goal 4 of SDG, and to replace existing indicators which have achieved optimal target, the PGI has been revised and renamed as PGI 2.0.
Sustainable Development Goal 4 (SDG4) adopted by India in 2015 seeks to ensure inclusive and equitable quality education and promote lifelong learning opportunities for all by 2030.
- Aim - To assess the relative performance of all the State/UTs in a uniform scale to encourage State/UTs to perform better.
- Ministry - Department of School Education and Literacy (DoSE&L), Ministry of Education.
- The PGI 2.0 is completely aligned with Unified District Information System for Education Plus (UDISE +), National Achievement Survey (NAS), PM POSHAN portal, PRABAND portal and Vidyanjali Portal data Outcomes.
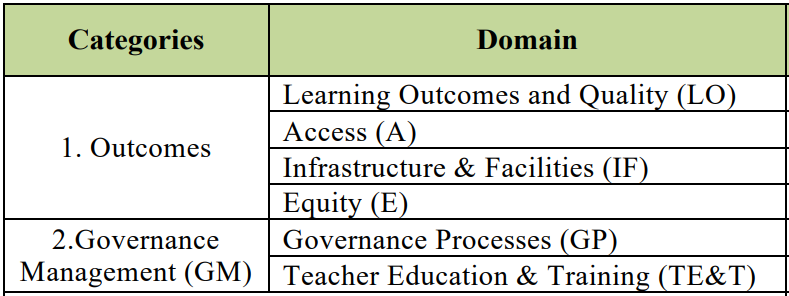
- Categories - The PGI 2.0 is constructed based on 73 indicators grouped in to 2 Categories viz, Outcomes and Governance & Management.
- It further contains 6 domains.
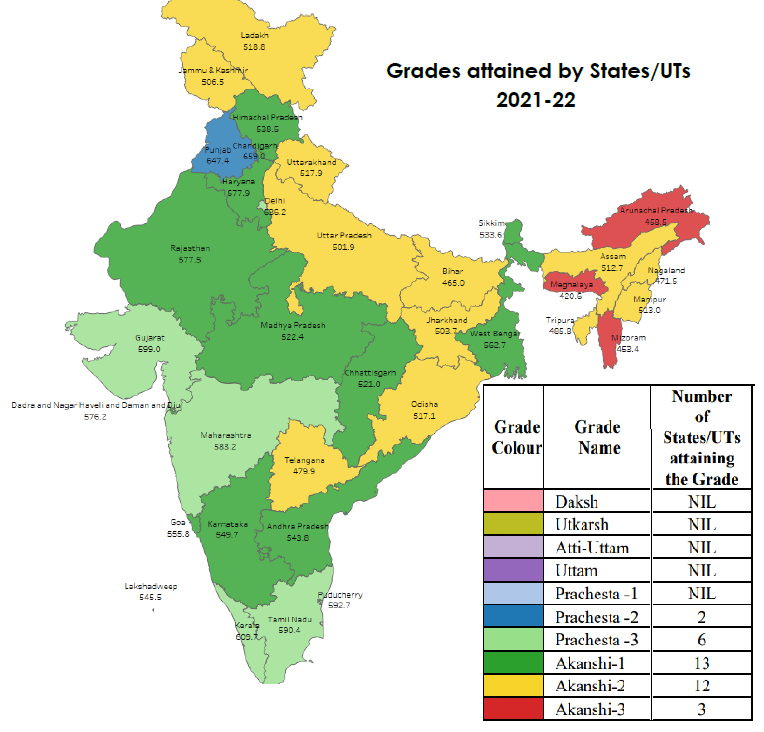
- PGI-D grades the State/UT into 10 grades – Daksh, Utkarsh, Ati-Uttam, Uttam, Prachesta-1, Prachesta-2, Prachesta-3, Akanshi-1, Akanshi-2, Akanshi-3.
- Highest achievable Grade – Daksh (Districts scoring more than 90% of the total points in that category or overall).
- Lowest grade - Akanshi-3 (scores upto 10% of the total points)
- None of the States/UTs has attained the highest Grade i.e., Daksh.
- The top-most grade attained in PGI 2.0 is Prachesta – 2 by Punjab and Chandigarh.
References
- PIB | Report on Performance Grading Index 2.0
- PGI Report | 2021-2022
Report Fish Disease App
To further strengthen the farmer based reporting of diseases, Report Fish Disease App has been developed under the National Surveillance Programme for Aquatic Animal Diseases (NSPAAD).
- To further strengthen the disease surveillance and farmer-based reporting, an app named Report Fish Disease was launched.
- It was launched by the Ministry of Fisheries, Animal Husbandry and Dairying.
- The farmers can report disease cases in finfish, shrimps and molluscs on their farms.
- The app will be a central platform for connecting fish farmers, field-level officers and fish health experts.
- Through this app each disease case in aquatic animals are reported, investigated and scientific advice are provided.
National Surveillance Program for Aquatic Animal Diseases (NSPAAD)
- Launch Year – 2013.
- Funding - National Fisheries Development Board, (NFDB), Hyderabad,
- Implementation - ICAR National Bureau of Fish Genetic Resources (NBFGR) Lucknow.
- I phase - This programme was initiated in 14 states of aquaculture importance and involved 24 collaborating centres.
- II phase – Launched in 2023 for a period of 3 years.
Reference
PIB | Mobile App developed under PMMSY
Majorana Fermion
Recently researchers at Microsoft figured out a way to create an elusive kind of particle that could potentially revolutionize quantum computing namely Majorana zero modes.
- Majorana Fermions - All subatomic particles that make up matter are called fermions.
- In other words, only fermions can make up matter.
- Fermions that are their own antiparticles are called Majorana fermions.
Antiparticle is a subatomic particle having the same mass as a given particle but having an opposite electric or magnetic property.
- Majorana Zero Mode - All particles have four quantum numbers associated with them.
- No two particles in the same system can have the same four quantum numbers. (The numbers are together like each particle’s ID).
- To be a fermion, one of the above said numbers, called the quantum spin, has only half-integer values, like ½, 3/2, 5/2, etc.
- Any particle, even something as large as an entire atom, can be a fermion, if its total quantum spin has a half-integer value.
- Bound states - Two particles that are bound to each other in some way can be a fermion, if their total quantum spin have a half-integer value.
- Most of the rules that apply to single fermions will also apply to these pairs, or bound states.
- When these bound states are their own antiparticles, they are called as Majorana zero modes.
- Majorana zero modes can be used to realise the more powerful topological quantum-computing.
Application of Majorana Zero modes
- Quantum computer - It uses individual electrons as qubits – its fundamental units of information.
- Information can be encoded in some property of each electron, like its spin.
- Then, the computer manipulates that information by having the electrons interact with each other according to the quirky rules of quantum mechanics.
- These quirks are what make quantum computers better than classical computers.
- But quantum computers are very fragile and if someone tap a finger on quantum computer table it could lose its quantummy abilities. i.e it may decohere.
- Majorana zero mode - It is composed of two entities electron and hole.
- A hole is a point where there could be an electron but isn’t and it effectively has a positive charge.
- A quantum computer made with qubit as Majorana zero mode and can be encoded with information onto some property of the mode.
- If it is configured with the two entities (electron and hole) apart and keep them at a distance from each other, physicists have found that even if one of the entities is disturbed, the overall qubit doesn’t decohere, and continues to protect the encoded information.
- A popular example of a system that could give rise to Majorana zero mode is a topological superconductor.
- To be a Majorana zero mode, any bound state should satisfy 2 conditions
- It should obey the Dirac equation
- It should be its own antiparticle
References
The Hindu | Microsoft’s planned quantum supercomputer
Nature | Majorana fermions
Candida Auris
Recently, Candida auris, a drug-resistant fungus that was identified as a global threat was found in hospitalised stray dogs in Delhi.
- About - Candida auris is an emerging multidrug-resistant oval-shaped fungus causing life-threatening outbreaks.
The World Health Organization has declared Candida auris as one of the world's 4 'critical priority' fungal pathogens.
- Origin - First reported in Japan in 2009, C. auris has since spread all over the world.
- It grows as yeast and causes candidiasis in humans.
- The fungus is hard to identify with standard laboratory methods and can be misidentified in labs without specific technology.
- Infections Caused - C. auris has caused bloodstream infections, wound infections, and ear infections.
- It also has been isolated from respiratory and urine specimens, but it is unclear if it causes infections in the lung or bladder.
- Spread - C. auris can spread in healthcare settings through contact with contaminated environmental surfaces or equipment, or from person to person.
- Treatment - Most C. auris infections are treatable with a class of antifungal drugs called echinocandins.
- However, some C. auris infections have been resistant to all 3 main classes of antifungal medications, making them more difficult to treat.
- In this situation, multiple classes of antifungals at high doses may be required to treat the infection.
- Findings - Report documents for the first time the isolation of live C. auris culture from an animal source.
- Overall, 4 of the 87 dogs (4.5%) contained evidence of C. auris infection or colonisation in their ear and on the surface of their skin.
- The recent finding suggests pets could act as reservoirs for superbugs, potentially transmitting infections to humans.
References
The Hindu | Drug-resistant fungus- Candida auris
Economic Times | Evidence of superbug found in Delhi's stray dogs
News-Medical.Net | Drug-resistant pathogen found in ears of stray dogs
Biosimilar Guideline (2016)
Health activists and patient groups seek revision of existing Biosimilar Guideline for increased access to critical drugs.
- Biologics – Biologics are medicinal products which are mainly composed of living tissues or cells.
- It mainly include vaccines, blood and blood components, gene therapy, tissues and recombinant therapeutic proteins.
- Biosimilar – A biologic which is found similar to another biologic is called a biosimilar (similar biologic).
- It is a medicine that is very close in structure and function to a biologic medicine and is safe and effective treatment options for many illnesses including arthritis, kidney conditions, and cancer.
- They increase access to lifesaving medications at potentially lower costs.
Biosimilar Guideline (2016)
- Prepared by- Central Drugs Standard Control Organization (CDSCO), Ministry of Health & Family Welfare.
- Aim – To address the regulatory pathway regarding manufacturing process and safety, efficacy and quality aspects for similar biologics.
- Features - A ‘similar biologic’ can only be developed against an authorized reference biological that has been approved using a complete data package in India.
- If the reference biological is not authorized in India, it should have been approved/licensed and marketed in an ICH (International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use) country.
- The Draft Guidelines document the eligibility criteria for conducting clinical trials on biosimilars.
- Phase III trials on biosimilars should include minimum 100 patients for evaluation, whereas Phase IV trials need at least 200 evaluable patients.
- As other drugs and formulations, the biosimilars are allowed to be manufactured and marketed after the patent of the original drug or product expires.
- Regulations and Guidelines
- The Similar Biologics are regulated as per the
- Drugs and cosmetics Act, 1940,
- Drugs and cosmetics Rules, 1945 and
- Rules for the manufacture, use, import, export and storage of hazardous microorganisms/ genetically engineered organisms or cells, 1989.
- Competent Authorities
- Institutional BioSafety Committee (IBSC)
- Review Committee on Genetic Manipulation (RCGM)
- Genetic Engineering Appraisal Committee (GEAC)
- Central Drugs Standard Control Organization (CDSCO)
- Data Requirements for Preclinical Studies
- The applicant has to comply with the RCGM requirements like demonstration of consistency of the process and product, product characterization and product specifications.
- Data Requirements for Clinical Trial Application
- Besides the information submitted in the preclinical application, the applicant has to submit application for conduct of clinical trial as per the CDSCO guidance for industry, 2008.
- Archiving of Data / Retention of Samples
- The applicant should archive all the data (quality, preclinical and clinical documentation) for a period of at least 5 years after marketing approval by competent authority in India.
References
The Hindu | Revision of Biosimilar Guideline (2016) needed
Cliniexperts | Draft Guidelines 2016

