Trachoma Free India
WHO declares that India has eliminated Trachoma as a public health problem in 2024.
- Trachoma – It is a bacterial infection that affects the eyes.
- World Health Organisation has termed it as a neglected tropical disease.
- Caused by – Bacterium Chlamydia Trachomatis.
- Transmission – It is contagious, spreading through contact with the eyes, eyelids, nose or throat secretions of infected people.
- Infection – Repeated infections in childhood lead to scarring of the inner side of the upper eyelids, resulting in inward turning of the eyelid margin, with the eyelashes touching the eyeball.
- If left untreated it causes irreversible blindness.
- Spread – Globally, 150 million people are affected and 6 million of them are blind or at risk of visually disabling complications.
- Susceptible population – It is found in underprivileged communities living in poor environmental conditions.

Elimination in India
- India – It was amongst the leading cause of blindness in the country during 1950-60.
- Measures by India
- NTCP – National Trachoma Control Program in 1963
- NPCB –National Program for Control of Blindness
- NPCBVI – National Programme for Control of Blindness & Visual Impairment in 1976
- WASH - water, sanitation and hygiene
- In 1971, blindness due to Trachoma was 5% and today, owing to the efforts under the NPCBVI, it has come down to less than 1%.
- WHO SAFE strategy – SAFE stands for adoption of surgery, antibiotics, facial hygiene, environmental cleanliness etc.
- Swachh Bharat Mission and Jal Jeevan Mission
The National Trachomatous Trichiasis (TT only) Survey was carried out in 200 endemic districts of the country under NPCBVI from 2021-24, which was a mandate set by WHO in order to declare that India has eliminated Trachoma as a public health problem.
- Elimination –
- In 2017, India was declared free from infective Trachoma
- In 2024, India has eliminated Trachoma as a public health problem.
- India becomes the 3rd country in the South-East Asia Region.
India joins Nepal and Myanmar in the WHO South-East Asia Region and 19 other countries globally that have previously achieved this feat.
- Recognition – An official Certification was handed over to Ministry of Health and Family Welfare by the WHO.
Reference
- PIB| Trachoma Free India
- WHO| Initiatives by India to Eliminate Trachoma
INS Nirdeshak
Recently, a survey vessel Nirdeshak (Yard 3026) was delivered to the Indian Navy.
- It is 2nd of 4 Survey Vessel (Large) ships, steered by the Indian Navy’s Warship Design Bureau.
- Built at – Garden Reach Shipbuilders & Engineers (GRSE), Kolkata
The 1st ship of the class, INS Sandhayak was commissioned early in 2024.
- Aim – It aims for full scale coastal and deep-water hydrographic survey of port/ harbour approaches and determination of navigational channels/ routes.
- Features – It displaces about 3400 tons and overall length is 110 meters and is powered by 2 diesel engines.
- It can achieve speeds in excess of 18 knots.
- It is fitted with state-of-the art hydrographic equipment such as
- Data acquisition and processing system
- Autonomous underwater vehicle
- Remotely operated vehicle
- DGPS long range positioning systems
- Digital side scan sonar, etc.
- Role – It will collect oceanographic and geophysical data for defence and civil applications.
- Importance – It has an indigenous content of over 80% by cost.
- It is a reassurance on impetus towards ‘Aatmanirbhar Bharat’.
- It is a tribute to the collaborative efforts of a large number of stakeholders, MSMEs and the Indian industry in enhancing the maritime prowess of India in the Indian Ocean Region.
Reference
PIB| Launch of Survey Vessel Ship Nirdeshak
Combination Therapeutic Implant
Scientists at Institute of Nano Science and Technology (INST) have developed & tested an indigenous intra-operative combination treatment consisting of drug and metal-based nanomedicine.
- Need – Surgery and chemotherapy are inevitable in managing solid tumours but localised tumour reappear.
- While Nano technological tools can reduce toxicity, there is an issue of the adsorption of host serum proteins over the surface of nanoparticles.
- Combination therapeutic implant – It consists of metal-based nanomedicine reinforced with patient derived blood clotting components.
- Working principle – The components are stabilized by patient derived serum protein corona termed as Nano-Micro-Sera (NMS).
- They are reinforced into autologous fibrin to aid in the post-surgical management of locally recurrent tumors.
- Usage – The autologous hybrid fibrin glue exhibited superior synergy and outcomes in suppressing recurrent breast tumors.
- Thus it reduces localised tumour recurrence post-surgery.
- Applications – It can be used to fabricate a therapeutic kit that can generate this autologous hybrid implant which might be beneficial to marginalised cancer patients.
|
Protein Corona
|
- It is a dynamic multilayer protein structure on the surface of Nanoparticle, formed by the rapid adsorption and accumulation of various proteins (such as albumin, apolipoprotein, and fibrinogen) after entering the intercellular environment.
- Importance – It has been recently established as a molecular fingerprint of a patient.
- It can be integrated into the basic design of nanoparticles for a futuristic personalized treatment strategy.
- They can be channelized towards generation of precision nanomedicines and diagnostic tools.
|
- Significance – It is an affordable methodology for localized post-surgical management.
Institute of Nano Science and Technology (INST) is an autonomous institute of Department of Science and Technology.
Reference
PIB| Combination Therapeutic Implant
Cancer Detection using Ultrasound
Scientists are working on a way to detect cancer with sound waves.
- Traditional method - The gold standard is a biopsy, where doctors extract a small piece of tissue or cells using a large needle from the part of the body where cancer is suspected to be present.
- In vitro tests can confirm if the tissue/cells are cancerous and, if so, what kind of cancer it is.
- Ultrasound method – Ultrasound waves are used in the blood sample of patients in detecting the presence of cancers in the body.
Ultrasound machines are used to take pictures of internal organs. The technology converts the sound waves reflected by surfaces inside the body to an image, just the way bats use ultrasound to sense their surroundings.
- Principle – High-energy ultrasound (at frequencies greater than those used in ultrasound scans) can break off a small piece of cancerous tissue into droplets.
- It release their contents into the bloodstream.
- The blood can be tested for biomarkers, certain biomolecules like DNA, RNA or proteins that are specific to cancer.
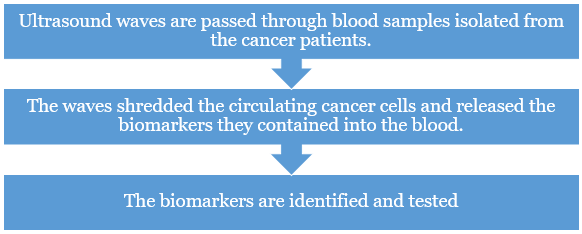
- Advantages - Ultrasound can enhance the levels of genetic and vesicle biomarkers in blood samples by over a 100-times.
Normally, when cancer progresses and spreads, cancer cells move to parts of the body other than their original site via the blood. But it is difficult to spot these cells in the blood because they’re very small in number.
- The blood samples can be used to detect specific cancer types and even the mutations they contain, which is currently undetectable in blood.
- Significance – The main advantage is its non-invasiveness, which will prevent patient discomfort.
- It could help clinicians avoid nearly half of all biopsies.
- It could be extended to monitoring cancer progression and treatment response.
|
Biomarker
|
- A biological characteristic that can be measured to indicate a normal or abnormal biological process, or a response to a treatment.
- Present in – Blood, urine, tissues, or other body fluids.
- Usage – It can be used to diagnose diseases, identify potential treatments and also to track disease progression.
|
Reference
The Hindu| Detection of Cancer using Ultrasound

