7667766266
enquiry@shankarias.in
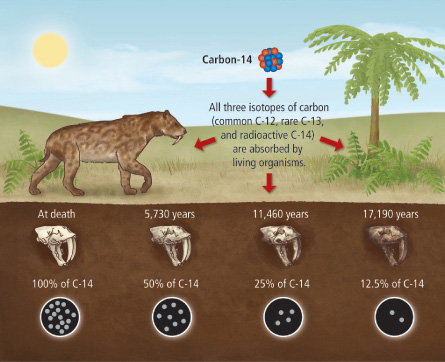
Recent study shows that Calcium-41 can be used the same way as Carbon-14 in carbon dating, but with several advantages.
References